Introductory Chemistry
Chapter 1
Science vs. Philosophy
Science was born out of philosophy. It was developed after it became obvious that philosophy alone could not fully explain the physical universe.
| Philosophers | Scientists |
|---|---|
| Observe Nature | Observe Nature |
| Explain the Behavior of Nature | Explain the Behavior of Nature |
| Communicate and Debate Ideas with other Philosophers. | Communicate and Debate Ideas with other Scientists. |
| Truth is revealed through Logic and Debate | Truth is revealed through Experimentation |
What Is Chemistry?
Chemists use the Scientific Method to discover the relationships between the particle structure of matter and the properties of matter we observe.
How does the microscopic properties of matter effect the macroscopic properties?
Structure Determines Properties
Everything is made of tiny particles called atoms and molecules.
Properties of a substance are determined by the type, amount, and interactions between these pieces.

The Scientific Method
A process for trying to understand nature by observing it and analyzing the way it behaves.
Observations are made to identify phenomenon to study and collect data.
Hypotheses are formed and tested through experimentation
Conclusions are drawn by analyzing data obtained from experiments.
These conclusions are used to confirm or reject the hypothesis

Observation
A way of acquiring information about nature.
The information obtained from observation is known as Data.
Some observations are simple descriptions about the characteristics or behavior of nature.
This is called qualitative data
“The soda pop is a liquid with a brown color and a sweet taste. Bubbles are seen floating up through it.”
Some observations compare a characteristic to a standard numerical scale.
This is called quantitative data
“A 240-mL serving of soda pop contains 27 g of sugar.”
Hypothesis
A tentative interpretation or explanation of your observations.
“The sweet taste of soda pop is due to the presence of sugar.”
A good hypothesis is one that can be tested to be proven wrong.
One test should be able to invalidate your hypothesis.
Experiments
Tests of hypotheses, laws, or theories.
Can you think of a way to test whether the sweet taste of soda pop is due to the presence of sugar?
Results either validate (confirm) or invalidate (deny) your ideas.
Invalidate = Discard or Modify
Many times experiments invalidate only parts of the hypothesis or theory, in which case the idea is modified.
Validate ≠ Proof your idea will always hold
Laws
Summary of observations that combines all past observations into one general statement.
Allows you to predict future observations.
Law of Conservation of Mass— “In a chemical reaction matter is neither created nor destroyed.”
What’s the Difference Between an Observation and a Law?
An observation tells you what happened in a single event.
A law summarizes all the observations, effectively telling you what you will observe in future events.
Theories
General explanation for the characteristics and behavior of nature.
Models of nature.
Ex. Dalton’s Atomic Theory, Theory of Gravity, Germ Theory
Can be used to predict future observations.
What’s the Difference Between a Hypothesis and a Theory?
A hypothesis is an explanation of a single or small number of observations.
A theory is an explanation that extends beyond individual observations to an understanding of the underlying causes for the way nature is or behaves.
What’s the Difference Between a Law and a Theory?
Laws answer the question “What” will happen.
Theories tell us "What" will happen but also “Why” it happens.
Theories allow to extend your predictions to a wider set of circumstances.
Applies to a Small Number of Events Applies to all Events Describes what happens Observation Law Describes why things happen Hypothesis Theory
Example from History
Why Do Some Things Burn?
Observations
Things would stop burning when placed in a closed container.
Many metals burn to form a white powder called calx.
Metals can be recovered from their calx by roasting it with charcoal.
Hypothesis
Phlogiston Theory is an Explanation of combustion proposed in early/mid-1700s.
Combustible substances contained a substance they called phlogiston.
When a substance burned it released all or some of its phlogiston into the air .
How Does Phlogiston Theory Explain the Observations?
When a substance is burned in the open, all the phlogiston is released.
When a substance is burned in a closed container, the phlogiston is released until it saturates the container, at which point the combustion stops.
A metal’s calx is what is left after it releases all its phlogiston.
When roasted with charcoal the calx reacquires phlogiston from the charcoal.
Charcoal is rich in phlogiston, that’s why charcoal burns.
Experiment
If phlogiston is lost when metals burn, then the metals should lose weight when burned.
Morveau’s experiments showed that when a piece of metal burned, the resulting calx weighed more than the original metal.
Do Morveau’s observations validate or invalidate the Phlogiston Theory?
If a calx is heated, it should remove phlogiston from the air as the calx is converted to the metal.
Lavoisier roasted many calx with a large lens and observed that material he called “fixed air” was released into the air.
Do Lavoisier’s observations validate or invalidate the Phlogiston Theory?
A Better Theory of Combustion
Lavoisier proposed an alternative theory of combustion.
When materials burn, they remove and combine with "fixed air" from the air.
Does Lavoisier’s idea explain all the previous observations?
How could you test Lavoisier’s idea?
Chapter 2
Exact Numbers
Sometimes you can determine an exact value for a quality of an object.
Often by counting.
Pennies in a pile.
Sometimes by definition
1 ounce is exactly 1/16 pounds.
From integer values in equations.
In the equation for the radius of a circle, the 2 is exact,
What Is a Measurement?
Quantitative observation.
Comparison to an agreed upon standard.
Every measurement has a number, a unit, and an indicated degree of uncertainty.
The unit tells you to what standard you are comparing your object.
The number tells you:
What multiple of the standard the object measures.
The uncertainty in the measurement.
Example
Scientists have measured the average global temperature rise over the past century to be 0.6 °C
°C tells you that the temperature is being compared to the Celsius temperature scale.
0.6 tells you that:
The average temperature rise is 0.6 times the standard unit of 1 degree Celsius.
The confidence in the measurement is such that we are certain the measurement is between 0.5 and 0.7 °C.
Scientific Notation
We commonly measure objects that are many times larger or smaller than our standard of comparison.
Writing large numbers of zeros is tricky and confusing.
Not to mention there may be a limit to the number of digits you can enter into your calculator
Each decimal place in our number system represents a different power of 10.
Scientific notation writes the numbers so they are easily comparable by looking at the power of 10.

Exponents
When the exponent on 10 is positive, it means the number is that many powers of 10 larger.
When the exponent on 10 is negative, it means the number is that many powers of 10 smaller.
To compare numbers written in scientific notation:
First compare exponents on 10.
If exponents are equal, then compare decimal numbers
Writing Numbers in Scientific Notation
Locate the decimal point.
Move the decimal point to obtain a number between 1 and 10.
Multiply the new number by 10n. Where n is the number of places you moved the decimal point.
If you moved the decimal point to the left, then n is positive; if you moved it to the right, then n is negative.
If the original number is 1 or larger, then n is positive .
If the original number is less than 1, then n is negative .
Example
12340
Locate the decimal point. 12340.
Move the decimal point to obtain a number between 1 and 10. 1.234
Multiply the new number by 10n . Where n is the number of places you moved the decimal point. 1.234 x 104
If you moved the decimal point to the left, then n is positive; if you moved it to the right, then n is negative . 1.234 x 104
Example
Locate the decimal point.
Move the decimal point to obtain a number between 1 and 10.
Multiply the new number by
If you moved the decimal point to the left, then
Example
The diameter of the sun is 1,392,000,000 m.
An atom's average diameter is 0.0000000003 m.
Writing a Number in Standard Form
Since the exponent is -6, make the number smaller by moving the decimal point to the left 6 places.
When you run out of digits to move around, add zeros.
Add a zero in front of the decimal point for decimal numbers.

The U.S. population in 2007 was estimated to be 301,786,000 people. Express this number in scientific notation.
Write the Following Numbers in Scientific Notation
Write the Following Numbers in Standard Form
Inputting Scientific Notation into a Calculator
We’re going to practice inputting the following into your calculator.
The number -1.23 × 10-3
Significant Figures
Reporting Measurements
Measurements are written to indicate the uncertainty in the measurement.
The system of writing measurements we use is called significant figures.
When writing measurements, all the digits written are known with certainty except the last one, which is an estimate.

Estimating the Last Digit
For instruments marked with a scale, you get the last digit by estimating between the marks.
If possible.
Mentally divide the space into 10 equal spaces, then estimate how many spaces over the indicator is.
What is the temperature reading on the thermometer to the correct number of digits?

Which Digits are Significant?
The non-placeholding digits in a reported measurement are called significant figures (sig figs) or significant digits.
Significant figures tell us the range of values to expect for repeated measurements.
The more significant figures there are in a measurement, the smaller the range of values. Therefore, the measurement is more precise.

Counting Significant Figures
All non-zero digits are significant.
1.5 has 2 significant figures.
Interior zeros are significant.
1.05 has 3 significant figures.
Trailing zeros after a decimal point are significant.
1.050 has 4 significant figures.
Leading zeros are NOT significant.
0.001050 has 4 significant figures.
Zeros at the end of a number without a written decimal point are ambiguous and should be avoided by using scientific notation.
If 150 has 2 significant figures, then 1.5 x 102, but if 150 has 3 significant figures, then 1.50 x 102.
Scientific Numbers are only written with Significant Digits. This is how you avoid ambiguity.
Significant Figures and Exact Numbers
Exact number’s value is known with complete certainty
They have an unlimited number of significant figures.
Determine the Number of Significant Figures, the Expected Range of Precision, and Indicate the Last Significant Figure
Rounding
When rounding to the correct number of significant figures, if the number after the place of the last significant figure is:
0 to 4, round down.
Drop all digits after the last significant figure and leave the last significant figure alone.
Add insignificant zeros to keep the value, if necessary.
5 to 9, round up.
Drop all digits after the last significat figure and increase the last significant figure by one.
Add insignificant zeros to keep the value, if necessary.
Example
Rounding to 2 significant figures.
2.34 rounds to 2.3
2.37 rounds to 2.4
2.349865 rounds to 2.3
0.0234 rounds to 0.023 or 2.3 × 10-2
0.0237 rounds to 0.024 or 2.4 × 10-2
0.02349865 rounds to 0.023 or 2.3 × 10-2
234 rounds to 230 or 2.3 × 102
237 rounds to 240 or 2.4 × 102
234.9865 rounds to 230 or 2.3 × 102
Multiplication and Division with Significant Figures
When multiplying or dividing measurements with significant figures, the result has the same number of significant figures as the measurement with the fewest number of significant figures.

Determine the Correct Number of Significant Figures for Each Calculation. Round and Report the Result.
Addition and Subtraction with Significant Figures
When adding or subtracting measurements with significant figures, the result has the same number of decimal places as the measurement with the fewest number of decimal places.

Determine the Correct Number of Significant Figures for Each Calculation. Round and Report the Result.
Both Multiplication/Division and Addition/Subtraction with Significant Figures
When doing different kinds of operations with measurements with significant figures, evaluate the significant figures in the intermediate answer, then do the remaining steps.
Follow the standard order of operations.
Please Excuse My Dear Aunt Sally.


Perform the Following Calculations to the Correct Number of Significant Figures
Units
Units tell the standard quantity to which we are comparing the measured property.
Without an associated unit, a measurement is without meaning.
Scientists use a set of standard units for comparing all our measurements.
So we can easily compare our results.
Each of the units is defined as precisely as possible.
Scientists generally report results in an agreed upon International System.
The SI System
Système International
| Quantity | Unit | Symbol |
|---|---|---|
| Length | meter | m |
| Mass | kilogram | kg |
| Time | second | s |
| Temperature | kelvin | K |
| Volume | liter (litre) | L |
Length
Measure of the one-dimensional distance an object covers.
The SI unit for length is a meter, about 3½ inches longer than a yard.
One ten-millionth the distance from the North Pole to the Equator
Distance between marks on standard metal rod in a Paris vault
Distance covered by a certain number of wavelengths of a special color of light

Mass
Measure of the amount of matter present in an object.
The SI unit is the kilogram (kg), about 2 lbs. 3 oz. It's important to note the base unit is not the gram.
Time
Measure of the duration of an event.
The SI units is the second (s)
1 s is defined as the period of time it takes for a specific number of radiation events of a specific transition from cesium-133.
Temperature
Measure of the average amount of kinetic energy,
The higher the temperature the greater the average kinetic energy
Heat (
Until they reach the same temperature.
Heat is exchanged through molecular collisions between the two materials.
SI System
All units in the SI system are related to the standard unit by a power of 10.
The power of 10 is indicated by a prefix.
The prefixes are always the same, regardless of the standard unit.
It is usually best to measure a property in a unit close to the size of the property.
It reduces the number of confusing zeros.
Example
| Prefix | Standard Form | Scientific Number |
|---|---|---|
| tera | ||
| giga | ||
| mega | ||
| kilo | ||
| hecto | ||
| deka | ||
| unit | ||
| deci | ||
| centi | ||
| milli | ||
| micro | ||
| nano | ||
| pico |
Example
| Volume | Volume in Liters |
|---|---|
Which of the Following Units Would Be Best Used for Measuring the Diameter of a Quarter?
kilometer
meter
centimeter
millimeter
megameters
Volume
Derived unit.
Any length unit cubed.

Measure of the amount of space occupied.
SI unit = cubic meter (m3)
Commonly measure liquid or gas volume in milliliters (mL).
1 L is slightly larger than 1 quart.
1 L = 1 dm3 = 1000 ml = 103 mL
1 ml = 0.001 L = 10-3 L
1 mL = 1 cm3
How to use Units
Always write every number with its associated unit.
Always include units in your calculations.
You can do the same kind of operations on units as you can with numbers.
cm × cm = cm2
cm + cm = cm
cm ÷ cm = 1
You can use units as a guide to problem solving
This is called dimensional analysis.
Conversions
Conversion factors are relationships between two units.
May be exact or measured.
Conversion factors generated from equivalence statements.
Arrange conversion factors so the starting unit cancels.
The starting unit should in the denominator of the conversion factor.
May string conversion factors.
So we do not need to know every relationship, as long as we can find something else the starting and desired units are related to :
Convert 1250 meters to miles. (1 mile = 1609.34 meters)
Convert 30.0 g to Ounces
Convert 30.0 mL to Quarts
An Italian recipe for making creamy pasta sauce calls for 0.75 L of cream. Your measuring cup measures only in cups. How many cups should you use?
Convert 2,659 cm to m
Convert 2,659 cm2 to m2
Mass and Volume
Two main characteristics of matter.
Cannot be used to identify what type of matter something is.
If you are given a large glass containing 100 g of a clear, colorless liquid and a small glass containing 25 g of a clear, colorless liquid, are both liquids the same stuff?
Even though mass and volume are individual properties, for a given type of matter they are related to each other!
Density
Ratio of mass to volume.
Its value depends on the kind of material, not the amount i.e. an intensive property
Solids = g/cm3 (g/ml)
Liquids = g/mL
Gases = g/L
Volume of a solid can be determined by water displacement—Archimedes Principle.
Density : solids > liquids > gases
Water is an exception
When volumes are equal, the more dense substance will be heavier .
When the mass of two samples is equal, the more dense substance will have smaller volume.
Heating causes objects causes objects to expand. Lowering their density.
Volume will increase
Mass will remain the same
In a heterogeneous mixture, the more dense object sinks.
Solve the density equation for mass and volume.
Platinum has become a popular metal for fine jewelry. A women places a ring on a balance and finds it has a mass of 5.84 grams. She then finds that the ring displaces 0.556 cm3 of water. Is the ring made of platinum? (Density Pt = 21.4 g/cm3)
What Is the Density of Metal if a 100.0 g Sample Added to a Cylinder of Water Causes the Water Level to Rise from 25.0 mL to 37.8 mL?
How much does 4.0 cm3 of lead (11.3 g/cm3) weigh?
The gasoline in a full automobile gas tank has a mass of 57.9 kg and a density of 0.752 g/cm3. What is the volume of the tank?
A 55.9 kg person displaces 57.2 L of water when submerged in a water tank. What is the density of the person in g/cm3?
Chapter 3
What is Matter?
Matter is defined as anything that occupies space and has mass
Even though it appears to be smooth and continuous, matter is actually composed of a lot of tiny little pieces we call atoms and molecules
Atoms are the tiny particles that make up all matter.
In most substances, the atoms are joined together in units called molecules
Classifying Matter by Physical State
Matter can be classified as solid, liquid or gas based on what properties it exhibits
These properties are the result of the arrangement of the atoms and molecules comprising a sample of matter.
| Phase | Shape | Volume | Compress | Flow |
|---|---|---|---|---|
| Solid | Fixed | Fixed | No | No |
| Liquid | Indefinite | Fixed | No | Yes |
| Gas | Indefinite | Indefinite | Yes | Yes |

Solid
The particles in a solid are packed close together and are fixed in position
though they may vibrate
The close packing of the particles results in solids being incompressible
The inability of the particles to move around results in solids retaining their shape and volume when placed in a new container; and prevents the particles from flowing
Some solids have their particles arranged in an orderly geometric pattern – we call these crystalline solids.
Salt and Diamonds are examples
Other solids have particles that do not show a regular geometric pattern over a long range. They are called amorphous solids
Plastic and Glass are examples

Liquids
The particles in a liquid are closely packed, but they have some ability to move around
the close packing results in liquids being incompressible
The ability of the particles to move allows liquids to take the shape of their container and to flow. However they don’t have enough freedom to escape and expand to fill the container.
Gases
In the gas state, the particles have complete freedom from each other
The particles are constantly flying around, bumping into each other and the container
In the gas state, there is a lot of empty space between the particles
on average
Because there is a lot of empty space, the particles can be squeezed closer together – therefore gases are compressible

Because the particles are not held in close contact and are moving freely, gases expand to fill and take the shape of their container, and will flow
Pure Substances vs. Mixtures

In a Pure Substance the entire sample is made of the same atoms or molecules.
All samples have the same properties
In Mixtures different samples may have components present in different percentages
Samples with varying composition will exhibit different properties.
Elements and Compounds
Substances which can not be broken down into simpler substances by chemical reactions are called elements.
Most substances are chemical combinations of elements. These are called compounds.
Compounds can be broken down into elements
Properties of the compound are not related to the properties of the elements that compose it
Elements
Smallest piece of an element is called an atom
There are subatomic particles, but these are no longer the element
Every sample of an element is made up of lots of identical atoms
118 known, of which about 91 are found in nature
The others are man made. Usually inside a particle accelerator.
There is a natural distribution of elements known as there abundance
The abundance and form of an element varies in different parts of the environment
Oxygen most abundant element (by mass) on earth and in the human body
Compounds
Smallest piece of a compound is called a molecule
Molecules are made of atoms
All molecules of a compound are identical
Each molecule has the same number and type of atoms
Composed of elements in fixed percentages
water is 89 %mass O and 11 %mass H
Billions of known compounds
Same elements can form more than one different compound
Water and hydrogen peroxide contain just hydrogen and oxygen
Carbohydrates all contain just C, H and O
Mixtures
Mixtures come in two forms homogeneous and heterogeneous.
Homogeneous Mixtures are uniform throughout
Appears to be one thing
Every piece of a sample has identical properties
Another sample with the same components may have different properties
Homogeneous mixtures are sometimes called solutions
Heterogeneous Mixtures are non-uniform
They contain different regions with different properties
| Pure Substances | Mixtures |
|---|---|
| All samples have the same physical and chemical properties | Different samples may show different properties |
| Constant composition; all samples have the same components in the same percentages. | Variable composition; samples made with the same pure substances may have different percentages |
| Homogeneous | Homogeneous or Heterogeneous |
| Separate components of a compound based on chemical properties | Separate into components based on physical properties |
| Temperature usually stays constant while melting or boiling | Temperature changes while melting or boiling because composition changes |
Properties of Matter
Physical Properties are the characteristics of matter that can be changed without changing its composition
Chemical Properties are the characteristics that determine how the composition of matter changes as a result of contact with other matter or the influence of energy
Some Physical Properties
mass
volume
density
phase
magnetic susceptability
specific heat
melting point
boiling point
volatility
taste
solubility
electrical conductivity
thermal conductivity
malleability
ductility
Some Chemical Properties
acidity
basicity
corrosiveness
reactivity
explosiveness
flammability
combustibility
reduction potential
Physical Changes
Physical Changes are changes in the properties of matter that do not effect its composition
Heating water raises its temperature. But it is still water.
Evaporating butane from a lighter
Dissolving sugar in water
Chemical Changes
Chemical Changes involve a change in a sample’s composition. A Chemical Reaction.
Rusting is iron combining with oxygen to make iron(III) oxide
Burning butane from a lighter changes it into carbon dioxide and water
Silver combines with sulfur in the air to make tarnish

Phase Changes are Physical Changes
Boiling = liquid to gas
Melting = solid to liquid
Subliming = solid to gas
Condensing = gas to liquid
Freezing = liquid to solid
Deposition = gas to solid
State changes require heating or cooling the substance
Evaporation is not a simple phase change, it is a solution process
Separation of Mixtures
Mixtures are separated based on different physical properties of the components
Physical Property Separation Technique Boiling Point Distillation Phase Filtration Surface Adhesion Chromatography Volatility Evaporation Density Centrifugation
Distillation and Filtration

Law of Conservation of Mass
This Law is attributed to Antoine Lavoisier
Matter is neither created nor destroyed in a chemical reaction
The total amount of matter present before a chemical reaction is always the same as the total amount after
The total mass of all the reactants is equal to the total mass of all the products
Total amount of matter remains constant in a chemical reaction
58 grams of butane burns in 208 grams of oxygen to form 176 grams of carbon dioxide and 90 grams of water
Energy
We have observed something that has neither mass or volume, Energy.
Energy is anything that has the capacity to do work
Even though Chemistry is the study of matter, matter is effected by energy
it can cause physical and/or chemical changes in matter
Law of Conservation of Energy
Energy can neither be created nor destroyed
The total amount of energy in the universe is constant – there is no process that can increase or decrease that amount
However we can transfer energy from one place in the universe to another, and we can change its form
When a piece of matter possesses energy, it can give some it to another object
All chemical and physical changes result in matter releasing or absorbing energy
Kinds of Energy
Kinetic Energy is energy of motion, or energy that is being transferred from one object to another
Potential Energy is energy that is stored
Electrical Energy is kinetic energy associated with the flow of electrical charge
Thermal Energy is kinetic energy associated with molecular motion
Light or Radiant Energy is kinetic energy associated with energy of subatomic particles called photons
Nuclear Energy is potential energy in the nucleus of atoms
Chemical Energy is potential energy in the attachment of atoms or because of their position
We use energy to accomplish all kinds of processes, but according to the Law of Conservation of Energy we don’t really use it up!
When we use energy we are changing it from one form to another
For example, converting the chemical energy in gasoline into mechanical energy to make your car move
In practice no process is 100% efficient. Some energy will be loss usually in the form of heat.
Units of Energy
calorie (cal) is the amount of energy needed to raise one gram of water by 1°C
1 food calorie or Calorie (cal)[Note the capital "C"} is 1,000 calories (cal) [Lower case "c"]l
1 Cal = 1000 cal = 1 kcal
Joule (J) is equal to the amount of work done when a force of 1 newton displaces a mass through a distance of 1 meter in the direction of the force applied.
It is the standard SI unit for energy
Kilowatt-hour (kWh) is the energy delivered by 1000 Watts of power over one hour.
Typically used when dealing with large amounts of energy

A candy bar contains 225 Cal of nutritional energy. How many joules does it contain?
Exothermic vs. Endothermic
A chemical change (reaction) can either release or absorb energy
Chemical reactions where energy is released are called exothermic
Chemical reactions where energy is absorbed are called endothermic
Energy is usually transferred in the form of heat

Classify each process as exothermic or endothermic.
a. gasoline burning in a car b. isopropyl alcohol evaporating from skin c. water condensing as dew during the night
Heat
Heat is the exchange of thermal energy between samples of matter
Heat flows from the matter that has high thermal energy to matter that has low thermal energy
Until they reach the same temperature
Heat is exchanged through molecular collisions between two samples
The Meaning of Temperature
Temperature is a measure of the average kinetic energy of the molecules in a sample
Not all molecules have in a sample the same amount of kinetic energy
A higher temperature means a larger average kinetic energy
Fahrenheit
The Fahrenheit Temperature Scale used as its two reference points the freezing point of concentrated saltwater (0 °F) and average body temperature (100 °F)
more accurate measure now set average body temperature at 98.6 °F
Room temperature is about 75 °F
Celsius
The Celsius Temperature Scale used as its two reference points the freezing point of distilled water (0 °C) and boiling point of distilled water (100 °C)
more reproducible standards
most commonly used in science
Room temperature is about 25 °C
Fahrenheit vs. Celsius
A Celsius degree is 1.8 times larger than a Fahrenheit degree
0 °C is 32 °F
Because the scales are offset from one another, we have a more complicated formula for converting between them.
Convert a temperature of
Convert a temperature of
A recipe requires an oven to be preheated to
The Kelvin Temperature Scale
Both the Celsius and Fahrenheit scales have negative numbers
The Kelvin scale is an absolute scale, meaning it does not allow for negative values.
0 K is called Absolute Zero. The lowest possible temperature.
All molecular motion would stop at 0 K
Absolute Zero is a theoretical value and has not yet been achieved in lab.
Kelvin vs. Celsius
The size of a “degree” on the Kelvin scale is the same as on the Celsius scale
that makes 1 K 1.8 times larger than 1°F
The 0 standard on the Kelvin scale is a much lower temperature than on the Celsius scale

Convert 37.8
Convert 465 K to Celsius
Convert 310 K to Fahrenheit
Energy and the Temperature of Matter
The amount the temperature of an object increases depends on the amount of heat energy added (
If you double the added heat energy the temperature will increase twice as much.
The amount the temperature of an object increases depends on its mass
If you double the mass it will take twice as much heat energy to raise the temperature the same amount.
Heat Capacity
Heat capacity is the amount of heat an object must absorb to raise its temperature 1°C
cal/°C or J/°C
Metals have low heat capacities
Thermal insulators high
Extensive quantity
Specific heat = heat capacity of 1 gram of the substance
cal/g°C or J/g°C
Waters specific heat = 4.184 J/g°C for liquid
1.000 cal/g°C
The larger a material’s specific heat is, the more energy it takes to raise its temperature.
Water’s high specific heat is the reason it is such a good cooling agent
like density, specific heat is a property of the type of matter
It can be used to identify the type of matter
it doesn’t matter how much material you have
Intensive quantity
| Substance | Specific Heat ( |
|---|---|
| Aluminum | 0.895 |
| Calcium | 0.656 |
| Carbon (diamond) | 0.508 |
| Carbon (graphite) | 0.708 |
| Copper | 0.377 |
| Gold | 0.129 |
| Iron | 0.448 |
| Lead | 0.129 |
| Silver | 0.712 |
| Water (l) | 4.184 |
| Water (s) | 2.03 |
| Water (g) | 2.02 |
Heat Gain or Loss by an Object
The amount of heat energy gained or lost by an object depends on 3 factors
The mass of the substance (m)
The sunstances Specific Heat Capacity (c)
The temperature changed, TF - Ti, or ΔT
Gallium is a solid metal at room temperature, but melts at 29.9°C. If you hold gallium in your hand, it melts from body heat. How much heat must 2.5 g of gallium absorb from your hand to raise its temperature from 25.0°C to 29.9°C? The heat capacity of gallium is 0.372 J/g°C
If 89 J of heat are added to a pure gold coin with a mass of 12 g, what is its temperature change?
A backpacker wants to carry enough fuel to heat 2.5 kg ofwater from 25 °C to 100.0 °C. If the fuel she carries produces 36 kJ of heat per gram when it burns, how muchfuel should she carry? (For the sake of simplicity, assume that the transfer of heat is 100% efficient.)
An iron nail with a mass of 12 g absorbs 15 J of heat. If the nail was initially at 28 °C, what is its final temperature?
Chapter 4
Atoms and Elements
Atoms are incredibly small, yet they compose everything.
Atoms are the pieces of elements.
Each has its own, unique kind of atom.
They have different structures. Therefore they have different properties.
Properties of the atoms determine the properties of the elements.
The Divisibility of Matter
Infinitely divisible
For any two points, there is always a point between.
Ultimate particle
Upon division, eventually a particle is reached which can no longer be divided.
Nothing exists axcept atoms and empty space; everything else is opinion. - Democritus 460 - 370 B.C.
Dalton’s Atomic Theory
Each Element is composed of tiny, indestructible particles called atoms.
All atoms of an element are identical.
They have the same mass, volume, and other physical and chemical properties.
Atoms combine in simple, whole-number ratios to form molecules of compounds.
Because atoms are unbreakable, they must combine as whole atoms.
The nature of the atom determines the ratios in which it combines.
Each molecule of a compound contains the exact same types and numbers of atoms.
Law of Constant Composition
Chemical formulas
Using compositions of compounds and assumed formulas, Dalton was able to determine the relative masses of all the atoms.
Dalton based his scale on H = 1 amu.
We now base it on 12C = 12 amu exactly.
amu = atomic mass unit.
Absolute sizes of atoms:
Mass of H atom= 1.67 x 10-24 g.
Volume of H atom = 2.1 x 10-25 cm3.
Charges
There are two kinds of charges, called positive (+) and negative (-).
Opposite charges attract.
Like charges repel.
Neutral objects either have no charge or equal amounts of opposite charges.

The Electron
Thompson Cathode Ray Tube
Work done by J. J. Thomson and others proved that the atom had pieces called electrons.
Thomson found that electrons are much smaller than atoms and carry a negative charge.
The mass of the electron is 1/1836th the mass of a hydrogen atom.
The charge on the electron is the fundamental unit of charge that we call –1 charge unit.
This brought him closer to our current model of an atom. But he still didn't have it quite right.
Rutherford’s Experiment
How can you prove something is empty? Put something through it.
Use large target atoms.
Use very thin sheets of target so they do not absorb the “bullet”.
Use very small particles as “bullet” with very high energy.
But not so small that electrons will effect it.
Rutherford used Gold Foil and alpha (α) particle radiation
Alpha particles have a mass of 4 amu & charge of +2 c.u.
Gold has a mass of 197 amu and is very malleable.

Over 98% of the alpha particles went straight through.
About 2% of the alpha particles went through, but were deflected by large angles.
About 0.01% of the alpha particles bounced off the gold foil.
“...As if you fired a 15”-canon shell at a piece of tissue paper and it came back and hit you.”
Rutherford’s Conclusions
Because almost all the particles went straight through, atoms are mostly empty space.
Because of the few particles that bounced back, atoms contain a dense particle that was small in volume, compared to the atom, but large in mass.
Because of the large deflections of some of the particles, he concluded that the dense particle was positively charged.
It would have to be to repel the positively charged alpha particles.

The Nuclear Model
The atom contains a tiny dense center called the nucleus.
The amount of space taken by the nucleus is only about 1 trillionth the volume of the atom.
The nucleus has essentially the entire mass of the atom. The electrons weigh so little they contribute practically no mass to the atom.
The nucleus is positively charged.
The amount of positive charge balances the negative charge of the electrons.
The electrons are dispersed in the empty space of the atom surrounding the nucleus.
Like water droplets in a cloud.
The Proton
Rutherford proposed that the nucleus had a particle that had the same amount of charge as an electron but opposite sign.
He called these particles are called protons.
Protons have a charge of +1 c.u. and a mass of 1 amu or 1.67262 × 10-27 kg.
Since protons and electrons have the same amount of charge, for the atom to be neutral, there must be equal numbers of protons and electrons.
The Neutron
How could beryllium have 4 protons stuck together in the nucleus? Shouldn’t they repel each other?
If a beryllium atom has 4 protons, then it should weigh 4 amu, but it actually weighs 9.01 amu!
Where is the extra mass coming from?
To answer these questions, Rutherford proposed that there was another particle in the nucleus.
Since this particle could not carry a charge he called it the neutron.
Neutrons have a mass of 1 amu or 1.67262 × 10-27 kg.
The Modern Atom
We now know atoms are composed of three main pieces
protons
neutrons
electrons.
The nucleus contains protons and neutrons.
The radius of the atom is about 105 times larger than the radius of the nucleus.

| Subatomic Particle | Mass (g) | Mass (amu) | Charge (c.u.) | Location | Symbol |
|---|---|---|---|---|---|
| Proton | 1.67262 × 10-24 | 1.0073 | 1+ | Nucleus | p+, H+ |
| Electron | 9.1 × 10-28 | 0.00055 | 1- | Orbital | e- |
| Neutron | 1.67493 × 10-24 | 1.0087 | 0 | Nucleus | n, n0 |
An Atom Has 20 Protons. Determine if Each of the Following Statements Is True or False?
A. If it is a neutral atom, it will have 20 electrons. B. If it also has 20 neutrons, its mass will be approximately 40 amu. C. If it has 18 electrons, it will have a net 2+ charge.
The Periodic Table
Mendeleev
Ordered elements by atomic mass.
Saw a repeating pattern of properties.
Periodic law
When the elements are arranged in order of increasing relative mass, certain sets of properties recur periodically?
Used pattern to predict properties of undiscovered elements.
Where atomic mass order did not fit other properties, he reordered by other properties.


Modern Periodic Table
Each element has a unique number of protons in its nucleus.
All carbon atoms have 6 protons in their nuclei.
The number of protons in the nucleus of an atom is called the atomic number.
Each element can be identified by its atomic number.
The elements are arranged on the Periodic Table in order of their atomic numbers.
Each element has a unique name and symbol.
The symbol is either one or two letters
Elements with similar chemical and physical properties are in the same column.
Columns are called Groups or Families.
Designated by a number
Rows are called Periods.
Each period shows the pattern of properties repeated in the next period.

What is the atomic number of boron, B?
What is the atomic mass of silicon, Si?
How many protons does a chlorine atom have?
How many electrons does a neutral neon atom have?
Will an atom with 6 protons, 6 neutrons, and 6 electrons be electrically neutral?
Will an atom with 27 protons, 32 neutrons, and 27 electrons be electrically neutral?
Will an Na atom with 10 electrons be electrically neutral?
Periodicity

Metals
Solids at room temperature, except Hg.
Reflective surface.
Conduct heat.
Conduct electricity.
Malleable..
Ductile.
Lose electrons and form cations in reactions.
About 75% of the elements are metals.
Lower left on the table.
Nonmetals
Found in all 3 states at standard temperature and pressure.
Poor conductors of heat.
Poor conductors of electricity.
Solids are brittle.
Gain electrons in reactions to become anions.
Upper right on the table.
Except H.
Metalloids
Show some properties of metals and some of nonmetals.
Also known as semiconductors.
Classify Each Element as Metal, Nonmetal, or Metalloid.
Xenon, Xe
Tungsten, W
Bromine, Br
Arsenic, As
Cerium, Ce
Groups


Charge and Ions
In a chemical change, the number of protons in the nucleus of the atom doesn’t change.
Radioactive and nuclear changes are an exception
Atoms in a compound are often electrically charged, these are called ions.
Atoms acquire a charge by gaining or losing electrons.
Never protons!
Ions with a positive charge are called cations.
Metals
More protons than electrons.
Form by losing electrons.
Ions with a negative charge are called anions.
Nonmetals
More electrons than protons.
Form by gaining electrons.
Chemically, ions are much different than the neutral atoms.
Anions are named by changing the ending of the name to –ide.
Cations are named the same as the metal.
The charge on a ion can often be determined from the group number on the periodic table.

Fill in the Table
| Ion | p+ | e- |
|---|---|---|
| Cl1- | ||
| K1+ | ||
| S2- | ||
| Sr2+ | ||
| Ca2+ |
Valence Electrons and Ion Charge
The highest energy electrons in an atom are called the valence electrons.
Metals form cations by losing their valence electrons to get the same number of electrons as the previous noble gas.
Main group metals.
Li+ has the same number of electrons as He
Al3+ has the same number of electrons as Ne
Nonmetals form anions by gaining electrons to have the same number of electrons as the next noble gas.
Cl- has the same number of electrons as Ar
Se2- has the same number of electrons as Kr
Isotopes
Soddy discovered that the same element could have atoms with different masses, which he called isotopes.
There are two isotopes of chlorine found in nature, one that has a mass of about 35 amu and another that weighs about 37 amu.
The atomic mass is a weighted average of the weights of all the naturally occurring atoms.
The atomic mass of chlorine is 35.45 amu.
The exact mass is the mass of a specific isotope
All isotopes of an element are chemically identical.
All isotopes of an element have the same number of protons and a different number of neutrons.
Isotopes of an element have different masses.
Isotopes are identified by their mass numbers.
Unlike the atomic mass or the exact mass, mass number is always a whole number Isotopes




Each isotope has a natural abundance based on the relative amount of the isotope found in nature
Natural abundance is the probability of finding a particular isotope in a sample of an element
Cl-35 makes up about 75% of chlorine atoms in nature, and Cl-37 makes up the remaining 25%.
What is the atomic mass of Neon?
| Isotope | Number of p+ | Number of n0 | Mass Number | Atomic Mass ( | Natural Abundance (%) |
|---|---|---|---|---|---|
| 10 | 10 | 20 | 19.992 | 90.48 | |
| 10 | 11 | 21 | 20.994 | 0.27 | |
| 10 | 12 | 22 | 21.991 | 9.25 |
How Many Protons and Neutrons Are in an Atom of
Gallium has two naturally occurring isotopes. Ga-69 with Mass 68.9256 Amu and Abundance of 60.11% and Ga-71 with Mass 70.9247 Amu and Abundance of 39.89%. Calculate the Atomic Mass of Gallium.
If Copper Is 69.17% Cu-63 with a Mass of 62.9396 Amu and the Rest Cu-65 with a Mass of 64.9278 Amu, Find Copper’s Atomic Mass.
Chapter 5
Molecules and Compounds
Compounds have chemical and physical properties distinct from their component elements.
Salt
Sodium—shiny, reactive, poisonous.
Chlorine—pale yellow gas, reactive, poisonous.
Sodium chloride—table salt.
Sugar
Carbon—pencil or diamonds.
Hydrogen—flammable gas.
Oxygen—a gas in air.
Law of Constant Composition
All samples of a pure substance contain the same elements in the same percentages (ratios).
The smallest piece of a compound is called a molecule.
Every molecule of a compound has the same number and type of atoms.
Since all the molecules of a compound are identical, every sample will have the same ratio of the elements.
Since all molecules of a compound are identical, every sample of the compound will have the same properties.
Mixtures have variable composition.
If we decompose water by electrolysis, we get 16.0 grams of oxygen to every 2.00 grams of hydrogen.
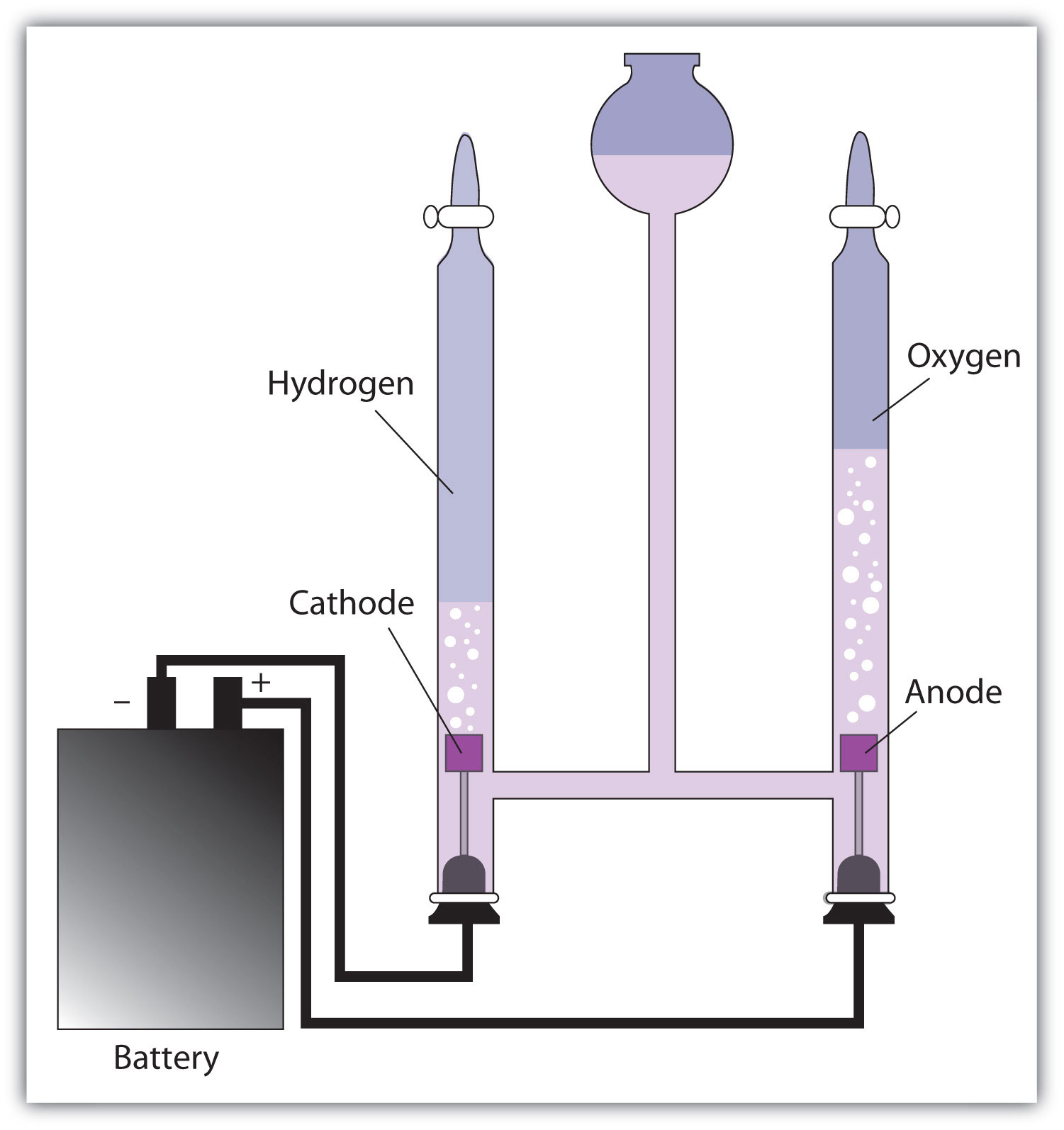
Water has a constant mass ratio of oxygen to hydrogen of 8.0
Show that Two Samples of Carbon Dioxide Are Consistent with the Law of Constant Composition.
| Sample | Carbon (g) | Oxygen (g) |
|---|---|---|
| 1 | 1.8 | 4.8 |
| 2 | 6.4 | 17.1 |
Show that Hematite Has Constant Composition if a 10.0 g Sample Has 7.2 g Fe and the Rest Is Oxygen; and a Second Sample Has 18.1 g Fe and 6.91 g O.
Polyatomics
Certain groups of atoms are bonded together to form what is called a polyatomic ion that acts as a single unit

Molecular Formulas Describe Compounds
We describe the compound by describing the number and type of each atom in the simplest unit of the compound.
Molecules or ions.
Each element is represented by its letter symbol.
The number of atoms of each element is written to the right of the element as a subscript.
If there is only one atom, the 1 subscript is not written.

Polyatomic groups are placed in parentheses.
If more than one.

Order of Elements in a Formula
Metals are written first.
Nonmetals are written in order
There are occasional exceptions for historical or informational reasons.
H2O, but NaOH .

Hematite is composed of four oxide ions for every three iron ions. What is the chemical formula for hematite?
Acetone molecules contain six hydrogen atoms, three carbon atoms, and one oxygen atom. What is it's chemical formula?
Determine the Total Number of Atoms or Ions in One Formula Unit of Each of the Following.
Mg(C2H3O2)2
Hg6(PO4)2
(NH4)3PO4
Structural Formulas
Structural formulas use lines to represent chemical bonds
Unlike molecular formulas, structural formulas demonstrate how the atoms in a molecular are connected.

Empirical Formulas
An empirical formula is the simplest whole-number ratio of atoms of each element in a compound.
| Molecular Formula | Empirical Formula |
|---|---|
| C6H6 | CH |
| C2O4H2 | CO2H |
| Al(NO3)3 | Al(NO3)3 |
Classifying Materials

Atomic Elements
Atomic elements have single atoms as their basic units.
Most elements fall into this category
Molecular Elements
Molecular elements do not normally exist in nature with single atoms as their basic units.
Smallest unit is a molecule.
Two or more nonmetals.
These elements usually exist as diatomic molecules.

Molecular Compounds
Molecular compounds are composed of two or more nonmetals.
The basic units are molecules.
For instance, H2O, CO2, C3H6O
Ionic Compounds
Ionic compounds are composed of one or more cations (+) paired with one or more anions (-)
Usually metals + nonmetals.
The basic unit of ionic compounds is the formula unit.
Smallest electrically neutral collection of ions
No real individual units, instead have a 3-dimensional array of cations and anions.
Classify Each of the Following as Either an Atomic Element, Molecular Element, Molecular Compound, or Ionic Compound.
Aluminum, Al.
Aluminum chloride, AlCl3.
Chlorine, Cl2.
Acetone, C3H6O.
Carbon monoxide, CO.
Cobalt, Co.
Writing Ionic Formulas
Ionic compounds are electrically neutral therefore there must be an equal number of positive and negative charges
We know sodium (Na) tends to form a cation with a 1+ charge
We also know that sulfur tends to form an anion with a 2- charge
To achieve an electrically neutral ionic compound, we will need two sodium ions for each sulfide ion.
Rules
Write the symbol for the metal cation and its charge.
Write the symbol for the nonmetal anion and its charge.
Charge (without sign) becomes subscript for the other ion.
Reduce subscripts to smallest whole-number ratio.
Check that the sum of the charges of the cation cancels the sum of the anions.

What Are the Formulas for Compounds Made from the Following Ions?
Potassium ion with a nitride ion
Calcium ion with a bromide ion
Aluminum ion with a sulfide ion
Magnesium ion with sulfite ion
Copper ion with a chloride ion
Ammonium ion with nitrate ion
Naming
Common Names
Some compounds have common names mostly due to historic significance
H2O = Water, steam, ice.
NH3 = Ammonia.
CH4 = Methane.
NaCl = Table salt.
C12H22O11 = Table sugar.
Ionic compounds.



Binary Ionic
Type 1

Type 2

When the anion is a polyatomic, the suffix is not changed
Write the name for the following ionic compounds
KCl
Na2O
CaBr2
CoF2
CuCl
Mg(NO2)2
Li2SO4
(NH4)3PO4
Al2(SO3)3
Write the formula for the following ionic compounds
Copper(II) Bromide
Iron(III) fluoride
Calcium Sulfate
Lithium Phosphate
Sodium Oxide
Molecular compounds.
2 or more nonmetals


Acids
Formula starts with hydrogen (H).
H2SO4, HBr
Sour taste
Though acids are molecular, they behave as ionic when dissolved in water.
May be binary or oxyacid.


Binary Acids
Binary acids have H+1 cation and nonmetal anion

Name the following binary acids
HF
HBr
HI
H2S
Oxyacids
Oxyacids have H+1 cation and polyatomic anion.

The names of acids containing oxyanions ending with -ite

The names of acids containing oxyanions ending with -ate

Name the following oxyacids
H2SO4
HNO3
HNO2
H3PO4
Write the chemical equation for the following acid
Sulfurous Acid
Hydrochloric Acid
Nitrous Acid
Chromic Acid
Formula Mass
The mass of an individual molecule or formula unit.
Also known as molecular mass or molecular weight.
Sum of the masses of the atoms in a single molecule or formula unit.
Calculate the Formula Mass for the following compounds
Chapter 6
Chemical Composition
Why Is Knowledge of Composition Important?
Everything in nature is either chemically or physically combined with other substances.
To know the amount of a specific element in a sample, you need to know what fraction of the sample it is.
Some Applications:
The amount of sodium in sodium chloride for diet.
The amount of iron in iron ore for steel production.
The amount of hydrogen in water for hydrogen fuel.
The amount of chlorine in freon to estimate ozone depletion.
The Mole
Counting Nails by the Pound
I want to buy a certain number of nails for a project, but the hardware store sells nails by the pound.
How do I know how many nails I am buying when I buy a pound of nails?
A hardware store customer buys 2.60 pounds of nails. A dozen nails has a mass of 0.150 pounds. How many nails did the customer buy?
1 dozen nails = 0.150 lbs. 12 nails = 1 dozen nails
A marble company produces three kinds of marbles. What is the average mass of the marbles? The company sells the marbles in bags of sixteen. What is the average mass of a bag of marbles in pounds?
| Color | Mass (oz) | Daily Production |
|---|---|---|
| Red | 2.1 | 1500 |
| Blue | 2.4 | 1300 |
| Orange | 1.9 | 1400 |
If we know the average mass of a particular number of atoms, we can use this information to convert the mass of an element sample to the number of atoms in the sample.
We can choose a clever quantity to make the units work out convienently
The quantity of atoms we will use is 6.022 x 1023 and we call this a mole.
1 mole = 6.022 x 1023 things.
Like 1 dozen = 12 things or 1 bag = 16 things
Avogadro’s number (
The mole is based on careful measurements made on the carbon-12 isotope
Mole = Number of things equal to the number of atoms in 12 g of C-12.
1 atom of C-12 weighs exactly 12 amu.
1 mole of C-12 weighs exactly 12 g.
A Silver Ring Contains 1.1 x 1022 Silver Atoms. How Many Moles of Silver Are in the Ring?
Calculate the Number of Atoms in 2.45 Mol of Copper.
Moles and Mass
The mass of one mole of atoms/molecules or is called the molar mass (
The molar mass (
The molar mass (
Calculate the Moles of Sulfur in 57.8 G of Sulfur.
Calculate the Mass of Carbon of 2.21 ⨯ 10-3 moles of Pencil Lead.
Calculate the number of moles of Carbon in 2.21 ⨯ 103 g of Pencil Lead.
Mass and Atoms
Using the number of moles allows us to convert between the mass of a sample (a measurable quantity) and the number of atoms or molecules.

How Many Aluminum Atoms Are in a Can Weighing 16.2 g?
What is the mass of 2.94 ⨉ 1022 atoms of Cu?
What is the mass of 2.94 ⨉ 1022 atoms of Cu?
Calculate the Mass of 1.75 Mol of H2O.
How Many Moles Are in 50.0 g of PbO2?
How Many Formula Units Are in 50.0 g of PbO2?
What Is the Mass of 4.78 x 1024 NO2 Molecules?
Chemical Formulas as Conversion Factors

If we know how many parts are in the whole unit, by counting the number of whole units, we can effectively count the parts.
Since we count atoms and molecules in mole units, we can find the number of moles of a constituent element if we know the number of moles of the compound.
Calculate the Moles of Oxygen in 1.7 Moles of CaCO3.
Find the Mass of Carbon in 55.4 g C10H14O.
Find the Mass of Sodium in 6.2 g of NaCl
Percent Composition
To determine the mass of a component from the mass of a compound you must go through the molar ratios.
However, the molar ratios and molar masses of the components for a specific compound is always the same.
We can be clever to make our math a little simpler
Find the Mass of Sodium in 6.2 g of NaCl using the percent composition.
The percent composition tells you the mass of a constituent element in 100 g of the compound.
Find the Mass Percent of Cl in C2Cl4F2.
The experimental mass analysis of the compound.
The percent composition of a sample can be measured directly through experimentation
The percentages may not always total to 100% due to rounding.
The percent composition data can be used to find the empirical formula of the compound
The simplest, whole-number ratio of atoms in a molecule.
The molecular formula is a multiple of the empirical formula.
Rules for finding an Empirical Formula from Percent Composition
Convert the percentages to grams. a. Skip if already grams.
Convert grams to moles. a. Use molar mass of each element.
Write a pseudoformula using moles as subscripts.
Divide all by smallest number of moles.
Multiply all mole ratios by number to make all whole numbers, if necessary. a. If ratio 0.5, multiply all by 2; if ratio 0.33 or 0.67, multiply all by 3, etc. b. Skip if already whole numbers after Step 4.
A laboratory analysis of aspirin determined the following mass percent composition. Find the empirical formula.
| Element | Percent Composition |
|---|---|
| C | 60.00 % |
| H | 4.48 % |
| O | 35.53 % |
A 3.24-g sample of titanium reacts with oxygen to form 5.40 g of the metal oxide. What is the formula of the oxide?
Determine the Empirical Formula of Stannous Fluoride, which Contains 75.7% tin and the Rest Fluorine.
Determine the Empirical Formula of Hematite, which Contains 72.4% Fe and the Rest Oxygen.*
Molecular Formulas From Empirical Formulas
The molecular formula is a multiple of the empirical formula.
To determine the molecular formula, you need to know the empirical formula and the molar mass of the compound.
Determine the Molecular Formula of Cadinene if it has a Molar Mass of 204 g/mol and an Empirical Formula of C5H8.
Benzopyrene has a Molar Mass of 252 g/mol and an Empirical Formula of C5H3. What is its Molecular Formula?
Determine the Molecular Formula of Nicotine, which has a Molar Mass of 162 g/mol and is 74.0% C, 8.7% H, and the Rest N.
Chapter 7
Chemical Reactions
Reactions involve chemical changes in matter resulting in new substances.
Chemical bonds are broken and formed to produce new molecules.
Molecules can combine to make bigger molecules.
Molecules can decompose into smaller molecules or atoms.
Atoms can be exchanged between molecules or transferred to another molecule.
Atoms can gain or lose electrons, turning them into ions.
Or changing the charge on ions that are already there.
Evidence of Chemical Reactions
Look for evidence of a new substance.
Permanent Visual clues.
Color change.
Precipitate formation.
Solid that forms when liquid solutions are mixed.
Gas bubbles.
Large energy changes.
Large Temperature Changes
Emission of light.
Other clues.
New odor.
Whooshing sound from a tube.
Permanent new phase. Evidence is Not Proof!
In order to be absolutely sure that a chemical reaction has taken place, you need to go down to the molecular level and analyze the structures of the molecules at the beginning and end.
Decide Whether Each of the Following Involve a Chemical Reaction.
Photosynthesis
Heating sugar until it turns black
Heating ice until it becomes a liquid
Digestion of food
Dissolving sugar in water
Burning paper
Chemical Equations
Short-hand way of describing a reaction.
Provides lots of information about the reaction.
Formulas of reactants and products.
Phases of reactants and products.
Relative numbers of reactant and product molecules that are required.
Can be used to determine masses of reactants used and products that can be made.
Symbols used in Chemical Equations
| Phase | Symbol |
|---|---|
| gas | ( g ) |
| liquid | ( l ) |
| solid | ( s ) |
| aqueous | ( aq ) |
| Energy | Symbol |
|---|---|
| heat | |
| light | |
| mechanical | |
| electrical |
| Symbol | Meaning |
|---|---|
| Indicates the direction of the reaction. From the reactants on the left to the products on the right. | |
| Indicates the reaction is capable of running in both directions (reversible) |
The Combustion of Methane
Methane gas burns to produce carbon dioxide gas and gaseous water.
Balancing Chemical Reactions
As written, there is not the same number of atoms on each side
This violates the Law of Conservation of Mass
O and H do not balance.


To correct this we have to adjust the stoichiometric coefficients
These indicate the number of each molecule participate in the reaction

This equation is balanced, meaning that there are equal numbers of atoms of each element on the reactant and product sides.
To obtain the number of atoms of an element, multiply the subscript by the coefficient.

Rules for Writing Balanced Chemical Equations
Write a skeletal equation by writing the formula of each reactant and product.
Count the number of atoms of each element on each side of the equation.
Polyatomic ions may often be counted as if they are one “element”.
Pick an element to balance.
If an element is found in only one compound on both sides, balance it first.
Metals before nonmetals.
Leave free elements until last.
Find the least common multiple (LCM) of the number of atoms on each side.
Multiply each count by a factor to make it equal to the LCM.
Use this factor as a coefficient in the equation.
If there is already a coefficient there, multiply it by the factor.
It must go in front of entire molecules, not between atoms within a molecule.
Recount and repeat until balanced.
When magnesium metal burns in air, it produces a white, powdery compound magnesium oxide. Write a balanced chemical equation for this reaction.
Write a skeletal equation
Count the number of atoms on each side.
Pick an element to balance.
Magnesium is already balanced so oxygen is the obvious choice.
Find the LCM of both sides
The least common multiple of 2 and 1 is 2.
Multiply each side by factor so it equals LCM.
Use factors as coefficients in front of the compound containing the element.
Recount
and Repeat—attacking an unbalanced element.
Recount—Mg not balanced now
Under appropriate conditions at 1000°C, ammonia gas reacts with oxygen gas to produce gaseous nitrogen monoxide and steam. Write a balanced chemical equation for this reaction.
When aluminum metal reacts with oxygen in the air, it produces a white, powdery compound called aluminum oxide. Write a balanced chemical equation for this reaction.
Acetic acid reacts with the metal aluminum to make aqueous aluminum acetate and gaseous hydrogen. Write a balanced chemical equation for this reaction.
Write a balanced chemical for the combustion of ethanol (C2H5OH).
Aqueous Solutions
Many times, the chemicals we are reacting together are dissolved in water.
Mixtures of a chemical dissolved in water are called aqueous solutions.
Dissolving the chemicals in water helps them to react together faster.
The water separates the chemicals into individual molecules or ions.
The separate, free-floating particles come in contact more frequently so the reaction speeds up.
We can predict whether or not a reaction will happen in aqueous media by considering various driving forces
“Forces” that drive a reaction:
Formation of a solid.
Formation of water.
Formation of a gas.
Transfer of electrons.
Dissociation
When ionic compounds dissolve in water, the anions and cations are separated from each other.
This is called dissociation.
Not all ionic compounds will dissolve in water!
When compounds containing polyatomic ions dissociate, the polyatomic group stays together as one ion.
Sodium chloride dissociates in water to form sodium cations and chloride anions.

Silver Nitrate dissociates in water to form silver cations and nitrate anions

Silver chloride does not dissolve or dissociate. It is insoluble in water.

Electrolytes
Electrolytes are substances whose water solution is a conductor of electricity.
All electrolytes have ions dissolved in water.
Strong electrolyte's molecules or formula units dissociate completely into ions.
Salts, some acids and bases
Weak electrolyte's molecules or formula units dissociate partially into ions.
Organic acids, alcohols
Nonelectrolyte's molecules or formula units do not dissociate into ions.
Sugars
Solubility
When Will a Salt Dissolve?
A compound is soluble in a liquid if it dissolves in that liquid.
NaCl is soluble in water, but AgCl is not.
A compound is insoluble if a significant amount does not dissolve in that liquid.
AgCl is insoluble in water.
Predicting whether a compound will dissolve in water is not easy.
A convenient way to do it is to do some experiments to test whether a compound will dissolve in water, then develop some rules based on those experimental results.
Solubility Rules


Determine if Each of the Following Is Soluble in Water
KOH
AgBr
CaCl2
Pb(NO3)2
PbSO4
Precipitation Reactions
Many reactions are done by mixing aqueous solutions of electrolytes together.
When this is done, often a reaction will take place from the cations and anions in the two solutions that are exchanging.
If the ion exchange results in forming a compound that is insoluble in water, it will come out of solution as a precipitate.
Process for Predicting the Products of a Precipitation Reaction
Write the formula for the reactants
Determine what ions each aqueous reactant has.
Exchange ions.
cations from one reactant with anions from the other.
Balance charges of combined ions to get formula of each product.
Balance the equation.
Determine solubility of each product in water.
Use the solubility rules.
If product is insoluble or slightly soluble, it will precipitate.
If neither product will precipitate, no reaction.
When an Aqueous Solution of Sodium Carbonate Is Added to an Aqueous Solution of Copper(II) Chloride, a White Solid Forms. Write the formulas of the reactants and Determine the ions present when each reactant dissociates.
Predict the Products and Balance the Equation
Ionic Equations
Equations that describe the chemicals put into the water and the product molecules are called molecular equations.
Equations that describe the actual dissolved species are called complete ionic equations.
Aqueous electrolytes are written as ions.
Soluble salts, strong acids, strong bases.
Insoluble substances and nonelectrolytes written in molecule form.
Solids, liquids, and gases are not dissolved, therefore, molecule form.
Notice that both the reactant and the product sides contain 2K+ and 2NO3- ions.
These are called spectator ions
Canceling the spectator ions results in the net ionic equation.
Summary
A molecular equation is a chemical equation showing the complete, neutral formulas for every compound in a reaction.
A complete ionic equation is a chemical equation showing all of the species as they are actually present in solution.
A net ionic equation is an equation showing only the species that actually participate in the reaction.
Write the Complete Ionic and Net Ionic Equation.
Acid/Base Reactions
Properties of Acids
Sour taste.
React with “active” metals, not noble metals.
I.e., Al, Zn, Fe, but not Cu, Ag or Au.
Corrosive.
React with carbonates, producing CO2.
Marble, baking soda, chalk, limestone.
React with bases to form ionic salts and often water.
Properties of Bases
Taste bitter
Caustic
Feel slippery
React with acids to form ionic salts.

Neutralization Reactions
The H+1 from the acid combines with the OH-1 from the base to make water. The cation from the base combines with the anion from the acid to make the salt.
The net ionic equation for an acid-base reaction is often
As long as the salt that forms is soluble in water.
Process for Predicting the Products of an Acid–Base Reaction
Determine what ions each aqueous reactant has.
Exchange ions.
cation from one reactant with anion from the other.
H+ combines with OH− to make water.
Balance charges of combined ions to get formula of the salt.
Balance the equation.
Determine solubility of the salt.
Use the solubility rules.
If the salt is insoluble or slightly soluble, it will precipitate.
Write the Molecular, Ionic, and Net-Ionic Equation for the Reaction of Aqueous Nitric Acid with Aqueous Calcium Hydroxide.
Complete and Balance these Acid–Base Reactions.
Gas Evolution Reactions
Reactions in which the driving force is the production of a material that escapes as a gas are called gas evolution reactions.
Some reactions form a gas directly from the ion exchange.
Other reactions form a gas by the decomposition of one of the ion exchange products into a gas and water.
Compounds that Undergo Gas Evolving Reactions
Metal Sulfides, M*n*S or MHS
Carbonates, M*n*CO3 or MHCO3
Sulfites, M*n*SO3 or MHSO3
Ammonium Salts, (NH4)*n*A
Process for Predicting the Products of a Gas-Evolving Reaction
Determine what ions each aqueous reactant has.
Exchange ions.
cation from one reactant with an ion from the other.
Balance charges of combined ions to get formula of each product.
Check to see if either product is H2S.
Check to see if either product decomposes. If so, rewrite as H2O(l) and a gas.
Balance the equation.
Determine solubility of other product in water.
When an Aqueous Solution of Sodium Sulfite Is Added to an Aqueous Solution of Nitric Acid, a Gas Evolves. Write the balanced chemical equation for this process.
Complete the Following Reactions.
Oxidation Reduction Reactions
Redox reactions occur when one chemical species loses one or more electrons to another.
The species that loses electrons in the reaction is oxidized.
The species that gains electrons in the reaction is reduced.
You cannot have one without the other.

In combustion, the O atoms in O2 are reduced, and the non-O atoms in the other material are oxidized.
Metals react with nonmetals to form ionic compounds.
The metal loses electrons and becomes a cation (oxidation).
The nonmetal gains electrons and becomes an anion (reduction).
The net result electrons are transferred from the metal to the nonmetal.
Example Metal with Nonmetal
In the reaction
The magnesium atoms are oxidized.
The chlorine atoms are reduced.
Example Combustion Reactions
Reactions in which O2(g) is a reactant are called combustion reactions.
Combustion reactions release lots of energy. They are exothermic.
Combustion reactions are a subclass of oxidation–reduction reactions.
In the following reaction
The magnesium atoms are oxidized.
The oxygen atoms are reduced.
Even though the following reaction does not involve ion formation, electrons are still transferred.
The carbon atoms are oxidized.
These are not charges, they are called oxidation numbers
They help us see the electron transfer.
The oxygen atoms are reduced.
Recognizing Redox Reactions
Any reaction where O2 is a reactant or a product is a redox reaction.
Any reaction between a metal and a nonmetal is redox.
Any reaction where electrons are transferred is redox.
When a free element gets combined into a compound, it will be either oxidized or reduced.
When a metal cation changes its charge
Oxidized if its charge increases or reduced if its charge decreases.
Decide Whether Each of the Following Reactions Is a Redox Reaction.
Classifying Reactions
One way is based on the process that happens.

Another scheme classifies reactions by what the atoms do.


Synthesis Reactions
Also known as composition or combination reactions.
Two (or more) reactants combine together to make one product.
Simpler substances combining together.
Example
Decomposition Reactions
A large molecule is broken apart into smaller molecules or its elements.
Caused by addition of energy into the molecule.
One reactant breaks into two or more products.
Example
Single Displacement Reactions
Reactions that involve one atom displacing another and replacing it in a compound.
Example
the atom Zn displaces H from the compound.
the Al atom displaces the Fe atoms
Na atoms displaces the H atoms

Double Displacement Reactions
Two ionic compounds exchange ions.
May be followed by decomposition of one of the products to make a gas.
Precipitation, acid–base, and gas evolving reactions are also double displacement reactions.
Example
Classify the Following Reactions as Synthesis, Decomposition, Single Displacement, or Double Displacement.
Chapter 8
Global Warming
Scientists have measured an average 0.6 °C rise in atmospheric temperature since 1860.
During the same period atmospheric CO2 levels have risen 25%.
The primary source of the increased CO2 levels are combustion reactions of fossil fuels we use to get energy.
1860 corresponds to the beginning of the Industrial Revolution in the U.S. and Europe.

Stoichiometry
The amount of every substance used and made in a chemical reaction is related to the amounts of all the other substances in the reaction.
Law of Conservation of Mass.
Balancing equations by balancing atoms.
The study of the numerical relationship between chemical quantities in a chemical reaction is called stoichiometry.
Making Sandwiches
A bologna sandwich requires one piece of bologna, one slice of cheese, and two slices of bread.
We can write a lot of relationships between these ingredients
The number of each ingredient required for one sandwich
The amount of bologna required when one of the other ingredients is consumed
The amount of cheese consumed when two slices of bread are used
We could even write the reciprocal of each of these relationships
We can use these relationships to find the number of ingredients needed to make five sandwiches
We can also use these relationships to calculate the number of sandwiches we can make given a certain number of ingredients
Making Water
Chemical Reactions work the same way
Keep in mind that this reaction is telling us that when 2 mol H2 reacts with 1 mol O2 , 2 mole of H2O is produced.
We can write several stoichiometric ratios
We can use these relationships to calculate the amount of water produced when 3.2 mole of O2 is reacted
Or to find out how much H2 is required to react with 3.2 mole O2
Or we could figure out how many moles of H2 would be required to produce 0.783 mole of H2O.
How Many Moles of NaCl Result from the Complete Reaction of 3.4 Mol of Cl2?
Measuring Amounts in the Lab
In the lab, our balances do not measure amounts in moles, unfortunately, they measure amounts in grams.
This means we must add two steps to each of our calculations
First convert the amount of each reactant to moles
Then convert the amount of product into grams.
How Many Grams of Glucose Can Be Synthesized from 58.5 g of CO2 in Photosynthesis?
How Many Grams of O2 Can Be Made from the Decomposition of 100.0 g of PbO2?
Limiting Reagents
Back to Sandwiches
What if you go to the kitchen and find 4 cheese slices, 11 slices of bread, and 3.5 slices of bologna.
How many sandwhiches could you make?
One way to think about this is to imagine how many sandwiches you could make assuming you have enough of the other ingredients.
Because bologna would produce the least number of sandwiches it is the limiting reagent
Bologna will be entirely consumed
There will be leftover cheese and bread
3.5 sandwiches is our Theroetical Yield
The amount of sandwiches we can make assuming we don’t drop one of the floor
We can calculate the amount of cheese and bread we will need to make 3.5 sandwiches
We can now calculate the amount of cheese and bread leftover
We can summarize our results in a table
| Bologna | Cheese | Bread | Sandwich | |
|---|---|---|---|---|
| Initial | 3.5 | 4 | 11 | 0 |
| Change | -3.5 | -3.5 | -7 | +3.5 |
| Final | 0 | 0.5 | 4 | 3.5 |
How Many Moles of Si3N4 Can Be Made from 1.20 Moles of Si and 1.00 Moles of N2 ?
What Is the Limiting Reagent and Theoretical Yield When 0.552 Mol of Al React with 0.887 Mol of Cl2?
Measuring Amounts in the Lab
In the lab, our balances do not measure amounts in moles, unfortunately, they measure amounts in grams.
This means we must add two steps to each of our calculations
First convert the amount of each reactant to moles
Then convert the amount of product into grams.
Percent Yield
No reaction goes all the way to completion
Some mass is loss (spilled, etc.) during any process
Therefore your actual yield will always be less than your theoretical yeld
%Yield is a metric to determine how close to the theretical yield you got
When 11.5 g of C Are Allowed to React with 114.5 g of Cu2O, 87.4 g of Cu Are Obtained. What is the % yield of this reaction?
How Many Grams of N2(g) Can Be Made from 9.05 g of NH3 Reacting with 45.2 g of CuO?
What Is the Percent Yield?
Enthalpy Change
We previously described processes as exothermic if they released heat, or endothermic if they absorbed heat.
The enthalpy of reaction (
At constant pressure.
Sign of Enthalpy Change
For exothermic reactions, the sign of the enthalpy change is negative
Thermal energy is produced by the reaction.
The surroundings get hotter.
For endothermic reactions, the sign of the enthalpy change is positive
Thermal energy is absorbed by the reaction.
The surroundings get colder.
Enthalpy and Stoichiometry
The amount of energy change in a reaction depends on the amount of reactants.
You get twice as much heat out when you burn twice as much CH4.
For the reaction
How Much Heat Is Associated with the Complete Combustion of 11.8 x 103 g of C3H8(g)?
How Much Heat Is Evolved When a 0.483 g Diamond Is Burned?